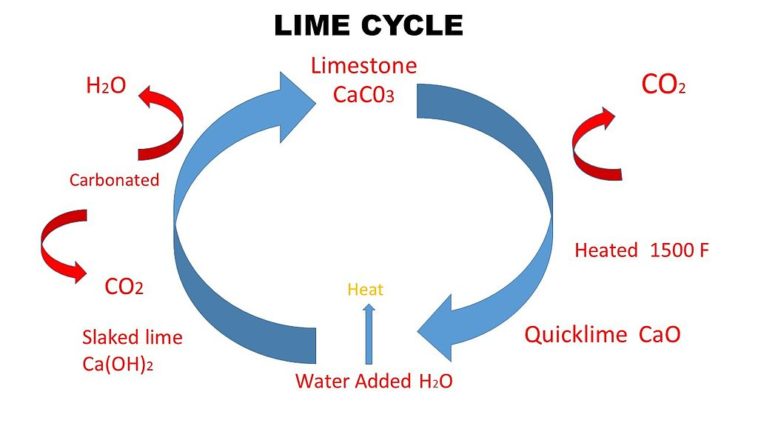
Quick lime is calcium oxide, CaO, in the solid state. Slaked lime is calcium hydroxide, Ca(OH)2, in the solid state. Lime water is a dilute solution of calcium hydroxide, Ca(OH)2, in water. Soda lime is a mixture of sodium hydroxide, NaOH, (also known as caustic soda) and calcium oxide, CaO, (also known as quicklime).
Moreover, What is lime water formula?
The formula for lime water is Ca(OH)2 and the chemical name for lime water is calcium hydroxide.
Secondly, Why do they cover bodies with lime?
It is used to capture the putrid scents of decaying flesh. Today lime is still used at mass grave sites to capture the scent of decay and keep soil pH high. Low pH soil is an indicator for a mass grave as the decomposition products are acidic and lower the soil pH.
Beside above What happens when water is added to quicklime? When quicklime is added to water, it forms slaked lime along with the evolution of heat. … Calcium oxide reacts with water to form calcium hydroxide, also called slaked lime.
In this way, What was lime used for in the past?
It was discovered that limestone, when burnt and combined with water, produced a material that would harden with age. The earliest documented use of lime as a construction material was approximately 4000 BC when it was used in Egypt for plastering the pyramids.
What happens to lime water when we exhale air into it?
exhaled air contain carbon doixide and when it comes contact with lime water, it turns milky. Turns lime water to milky because of the carbondioxide from the exhaled air. The carbon dioxide in our exhaled air reacts with the calcium hydroxide in the lime water to produce calcium carbonate which is milky in colour.
Contenus
14 Related Questions and Answers Found
Why do some gas bubbles passed through lime water when heating is first started?
Reason : When Carbon dioxide is passed through Lime water or Calcium Hydroxide, it turns milky due to the formation of the Precipitate of the insoluble milky Calcium Carbonate.
Can you drink lime water?
Drinking lime water improves digestion. Limes are acidic and they help saliva break down food for better digestion. Additionally, flavonoids in limes stimulate secretion of digestive juices. If you experience constipation, the acidity of limes can clear the excretory system and stimulate bowel activity.
Why do they cover dead bodies with sheets?
A sheet is draped over a deceased person’s body to provide privacy and to show respect. As a reporter myself, it’s a good thing to do as some reporters will not hesitate to snap photos if the person/story is newsworthy.
Does lime break down a body?
The actual effects of lime on the decomposition of human remains were studied by Schotsmans et al. (2012; 2014a;2014b) based on field and laboratory experiments. The results showed that lime retards the rate of decomposition if present in a burial environment, but does not stop it. …
Should I use lime when burying my dog?
It is recommended that the dead animal be covered with lime or similar material prior to being covered with soil. This will aid in decomposition and reduce the potential for odors. In areas of high groundwater, animals cannot be buried within three (3) feet of groundwater depth.
Is lime poisonous?
Because lime’s sole purpose is to increase the pH of acidic soil, it’s an incredibly alkaline substance. … Again, lime is widely considered to be non-toxic, accidentally consuming or breathing it in may cause some problems.
What happens chemically when quick lime?
When quicklime (calcium oxide) is added to water filled in a bucket, it reacts with water chemically to form slaked lime (calcium hydroxide) and produce heat energy. So, water in the bucket becomes hot as it is an exothermic reaction.
What happens when you burn quicklime?
Lime burning produces quicklime/calcium oxide (CaO) and carbon dioxide (CO2) which escapes into the environment. This reaction takes places at temperatures above 700°C. Quicklime is highly reactive and in contact with water it changes into slaked lime/ calcium hydroxide (Ca(OH)2).
Is lime harmful to humans?
Because burned and hydrated lime are caustic, extreme care should be used when applying these to your lawn. According to Virginia State University, calcitic and dolomitic lime are nontoxic to humans, wildlife and pets, which means they haven’t been found to cause illness or death when ingested.
Will limes eat through plastic?
Does lime eat through plastic? It will not harm plastic, but will ‘burn’ flesh even your throat or lungs if you breath the dust from it. The ‘burn’ is really a chemical harm done to the skin similar to a strong acid (‘only different’).
What is hydraulic lime used for?
Hydraulic limes set by hydrolysis, a reaction caused by water. It causes a faster and harder set, therefore these limes are more often used for exterior work, especially in exposed or damp conditions. Hydraulic limes are available as a bagged powder and in differing degrees of strength.
What happens when air is being passed into lime water with a Pichkari?
Air is exhaled through lime water and air is passed through lime water using pichkari. … Lime water will turn milky first when air is exhaled through lime water because exhaled air contains a greater concentration of carbon dioxide than present in air.
What is respiration How is it different from breathing?
Breathing and respiration are two completely different but interrelated body processes which assist body organs to function properly. Breathing is the physical process of exchanging gases whilst respiration is a chemical process which takes place at a cellular level and produces energy.
What is process of breathing?
The process of breathing, or respiration, is divided into two distinct phases. The first phase is called inspiration, or inhaling. When the lungs inhale, the diaphragm contracts and pulls downward. At the same time, the muscles between the ribs contract and pull upward.
What gas turns lime water into milky white?
Limewater as Indicator of Carbon Dioxide Gas. Description: Carbon dioxide gas from a cylinder is bubbled through limewater and calcium carbonate solid is formed causing the limewater to become cloudy.
Why the lime water turns milky?
Carbon dioxide reacts with calcium hydroxide solution to produce a white precipitate of calcium carbonate. Limewater is a solution of calcium hydroxide. If carbon dioxide is bubbled through limewater, the limewater turns milky or cloudy white.
Why should lime water be freshly prepared?
Why is freshly prepared lime water used in experiment to test the evolution of carbon dioxide? Freshly prepared lime water is used in the experiment to test the presence of CO2 because lime water turns milky when CO2 passes through it.
Editors. 25 – Last Updated. 30 days ago – Authors. 2