If you soak this egg shell in vinegar (which is about 4% acetic acid), you start a chemical reaction that dissolves the calcium carbonate shell. The acetic acid reacts with the calcium carbonate in the egg shell and releases carbon dioxide gas that you see as bubbles on the shell.
Moreover, What happens when you leave an egg in vinegar for 3 days?
If you soak an egg in vinegar the eggshell will absorb the acid and break down, or dissolve. The calcium carbonate will become carbon dioxide gas, which will go into the air. What is left is the soft tissue that lined the inside of the eggshell. It will bounce!
Secondly, What happens if you put an egg in coke for 24 hours?
After the Coca Cola and egg was left for a year, the soda reacted with the egg shell made up of calcium carbonate reacted with the acid and the result was surprising. … If this is the reaction of the egg shell and Coca-Cola, consider what it can do to your teeth and how it can erode and destroy the enamel of your teeth.
Beside above What liquid makes an egg bounce? The Science Behind It: The shell of an egg is made of calcium carbonate! When the egg is placed into the vinegar, you see bubbles, which is the chemical reaction of the acid within the vinegar reacting with the calcium carbonate of the egg shell to produce carbon dioxide.
In this way, What happens when you put an egg in water for 24 hours?
Leave the egg in the water for 24 hours. The water will migrate from the side of the membrane where water molecules are abundant (outside the egg) to the side where water molecules are less abundant. After 24 hours, the egg will be plump again.
What happens if you leave an egg in vinegar for too long?
What happens? Be careful, the eggshell will be a lot weaker! If you leave the egg in the vinegar for about 36 hours, eventually all the calcium carbonate will be dissolved by the acetic acid, leaving just the soft membrane and yolk behind.
Contenus
21 Related Questions and Answers Found
What happens when you put an egg in vinegar osmosis?
This is because the vinegar has a higher concentration of water than the inside of the egg. … To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup until both solutions have the same concentration of water. The outward movement of water causes the egg to shrivel.
What happens when you put an egg in Coke for 24 hours?
After the Coca Cola and egg was left for a year, the soda reacted with the egg shell made up of calcium carbonate reacted with the acid and the result was surprising. … If this is the reaction of the egg shell and Coca-Cola, consider what it can do to your teeth and how it can erode and destroy the enamel of your teeth.
What happens if you leave an egg in syrup?
Since the egg membrane is semi-permeable, water can move in but proteins cannot move out. If a naked egg is placed in the corn syrup the egg will shrink. This is also due to osmosis, but in the opposite direction. The corn syrup is mostly sugar.
Why does Coke dissolve egg shell?
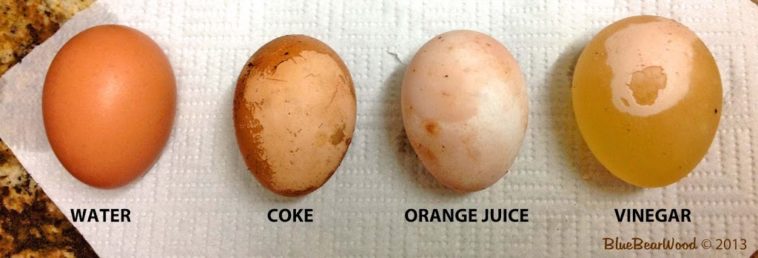
Diet coke and orange juice are also acids—why don’t they dissolve the egg shell too? Diet coke has a pH of 3.1, orange juice has a pH of 3.5, and vinegar has a pH of 2.5. … Therefore, it dissolves the eggshell much faster.
What happens if you put toothpaste on an egg?
What happens when you put toothpaste on an egg? The fluoride content in toothpaste keeps the eggshells safe from the acids present in black coffee and coke.
What happens when you put an egg in vinegar than corn syrup?
To reach equilibrium, water molecules move from the vinegar into the egg through the semi-permeable membrane. … Since the egg membrane is semi-permeable, water can move in but proteins cannot move out. If a naked egg is placed in the corn syrup the egg will shrink.
Does Coke dissolve egg shell?
The chemical reaction between the acetic acid in the cola and the calcium carbonate in the eggshell causes the shell to dissolve.
Is it OK to soak eggs in water?
Eggs are porous and have active bacteria on the outside, so they should not be dipped or soaked in soapy water, Coufal said. … Avoid using dish soap or scented cleaning solutions as they can affect the eggs’ taste. After washing, eggs should be rinsed with clean water that is slightly warmer than the wash water, he said.
What happens when you put an egg in pure water?
When you put the egg into pure water, some of the water molecules from the solution move into the egg and the egg swells up. … This happens because, while most of the water molecules flow out of the egg, after some time passes, water molecules pass into the egg at the same rate as they are flowing out.
What happens if you put an egg in salt water?
Saltwater is denser than fresh water because of its salt content. The egg will sink in the fresh water because it has greater density than the water. The egg will float in the salt water because when salt is added to water its density becomes greater than that of the egg. That makes the egg float.
What happens when you soak an egg in lemon juice?
Bubbles will form on the egg shell. The egg shell will begin dissolving and finally the mixture turns white. … There should still be a soft membrane surrounding the egg but no shell as this has dissolved into the lemon juice to make calcium citrate.
What happens when you put an egg in syrup?
When you put a naked egg in corn syrup, you are creating a situation where the egg membrane separates two solutions with different concentrations of water. The egg white is about 90% water; corn syrup is about 25% water. … So water migrates from inside the egg to outside the egg, leaving the egg limp and flabby.
What happens when you put an egg in salt water?
Saltwater is denser than fresh water because of its salt content. The egg will sink in the fresh water because it has greater density than the water. The egg will float in the salt water because when salt is added to water its density becomes greater than that of the egg. That makes the egg float.
Is vinegar hypertonic to an egg?
This is because the water in the vinegar can enter the egg through the membrane, moving from the higher water concentration in vinegar to the lower concentration in the egg. … This means that the egg will shrink in size. The corn syrup is a hypertonic liquid, ie. very concentrated with not much water compared to the egg.
Is salt water hypertonic to an egg?
An egg’s membrane will allow small molecules like water to pass through, but not large ones like salt. … The egg in the salt water shrunk. This is because the solution outside the egg is more concentrated, so the water flowed out from the dilute solution to the concentrated solution.
What happens if you put an egg in syrup?
When you put a naked egg in corn syrup, you are creating a situation where the egg membrane separates two solutions with different concentrations of water. The egg white is about 90% water; corn syrup is about 25% water. … So water migrates from inside the egg to outside the egg, leaving the egg limp and flabby.
What happens if you put an egg in bleach?
Bleach causes these proteins to unfold or to clump together. This clumping is the same kind of thing that happens when you heat an egg — the protein molecules in the egg solidify as they clump together. If you put bleach in water, it will kill bacteria and tend to lessen anything that might be coloring the water.
Editors. 26 – Last Updated. 4 days ago – Authors. 9